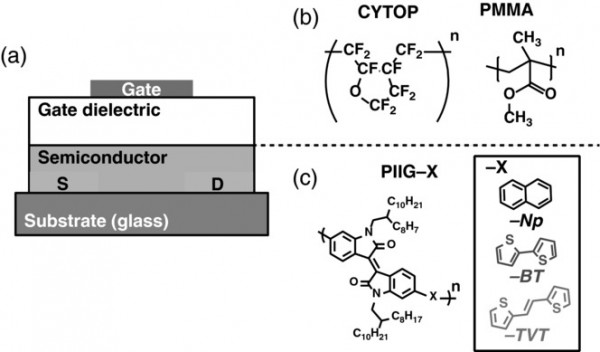
Effect of Donor Molecular Structure and Gate Dielectric on Charge‐Transporting Characteristics for Isoindigo‐Based Donor–Acceptor Conjugated Polymers
- 저자
- Won-Tae Park, Gyoungsik Kim, Changduk Yang*, Chuan Liu*, Yong-Young Noh*
- 저널명
- Advanced Functional Materials, 26, 26, 4695-4703 (2016)
- 년도
- 2016
- Link
- http://dx.doi.org/10.1002/adfm.201504908 810회 연결
[Abstract]
This study investigates the effect of the molecular structure of three different donor units, naphthalene (Np), bithiophene (BT), and thiophene–vinylene–thiophene (TVT), in isoindigo (IIG)-based donor –acceptor conjugated polymers (PIIG-Np, PIIG-BT and PIIG-TVT) on the charge carrier mobility of organic field-effect transistors (OFETs). The charge transport properties of three different IIG-based polymers strongly depend on donor units. PIIG–BT OFETs showed 50 times higher hole mobility (0.63 cm2 V−1 s−1) than PIIG–TVT and PIIG–Np ones of ≈ 0.01 cm2 V−1 s−1 with CYTOP dielectric though the BT units have less planarity than the TVT and Np units. The reasons for the different mobility in IIG-based polymers are studied by analyzing the energy structure by absorption spectra, calculating transport levels by density functional theory, investigating the in- and out-of-plane crystallinity of thin film by grazing-incidence wide-angle X-ray scattering, and extracting key transport parameters via low-temperature measurements. By combining theoretical, optical, electrical, and structural analyses, this study finds that the large difference in OFET mobility mainly originates from the transport disorders determined by the different microcrystal structure, rather than the intrinsic transport properties in isolated chains for different polymers.
- 이전글Enhanced hole extraction by interaction between CuI and MoO3 in the hole transport layer of organic photovoltaic devices 24.12.15
- 다음글High-Mobility Naphthalene Diimide and Selenophene-Vinylene-Selenophene-Based Conjugated Polymer: n-Channel Organic Field-Effect Transistors and Structure–Property Relationship 24.12.15